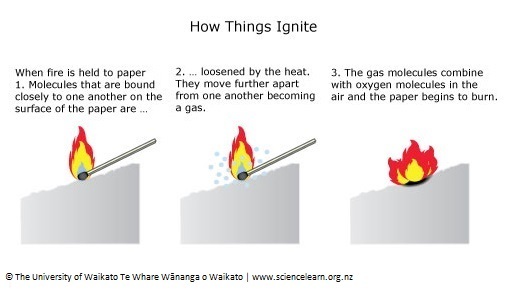
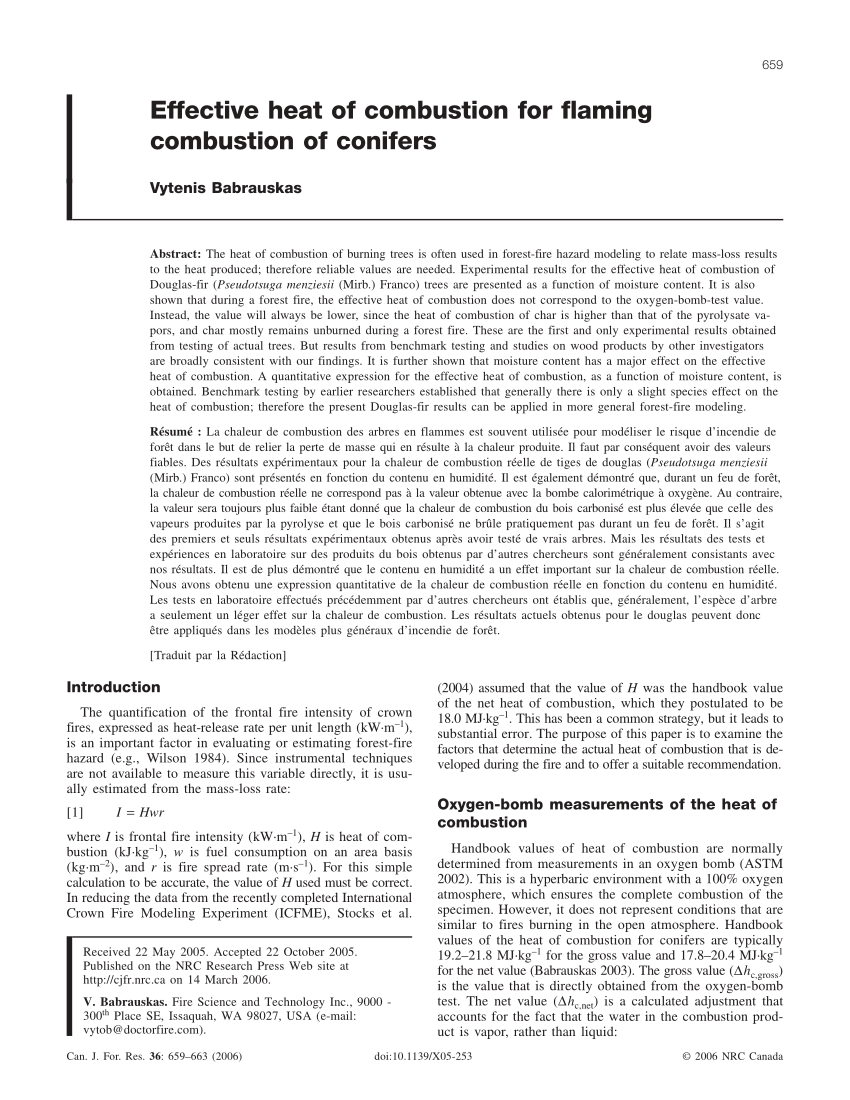
PDF) Relationship between heat of combustion, lignin content and burning weight loss | Jadwiga Fangrat - Academia.edu

The heat of combustion of carbon is 394 kJ/mol. The heat evolved in combustion of 6.023 × 10^22 atoms of carbon is:
Paper Heat Boiler Combustion Furnace Room, PNG, 1311x1365px, Paper, Area, Biomass, Boiler, Brand Download Free
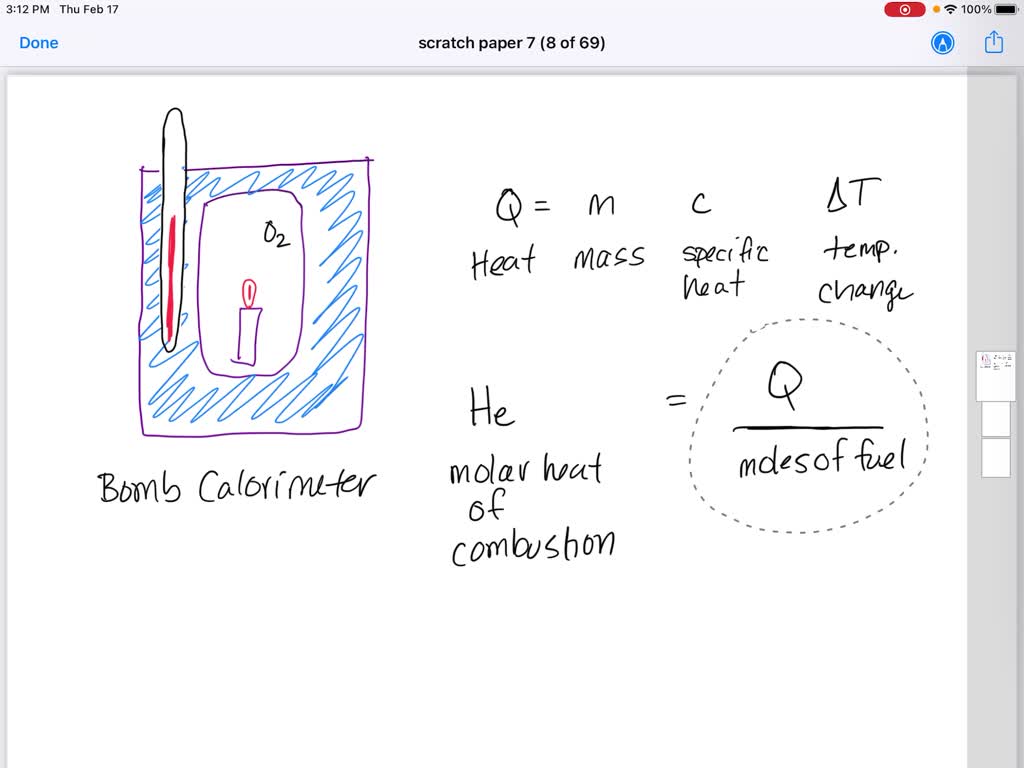
SOLVED: The quantity of heat released when a given amount of substance burns is called the heat of combustion and it is symbolized by ∆H comb. Paraffin, C 25 H 52, is
Heat of combustion and formation of cyanogen : Knowlton, J.W. : Free Download, Borrow, and Streaming : Internet Archive
Heats of Combustion of the Five-Carbon Fatty Acids and their Methyl and Ethyl Esters | The Journal of Physical Chemistry

Acceptable Range of Heat of Combustion for MSW HEAT OF COMBUSTION OF... | Download Scientific Diagram